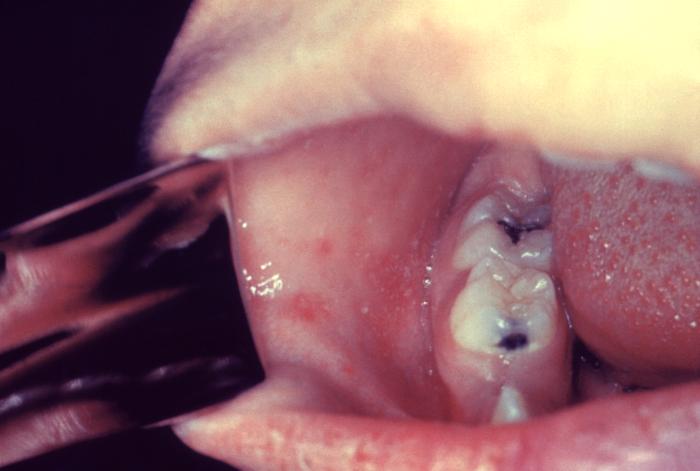
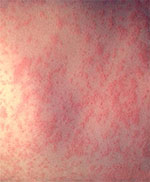
Note: Do not accept verbal reports of immunity
Acceptable presumptive evidence of immunity against measles includes ≥1 of the following
*Note: CDC addresses laboratory evidence of immunity and states the following
*People who have negative or equivocal results for measles IgG should be vaccinated or revaccinated. In some cases it is not possible to vaccinate a patient, and you may need to test them with a second line diagnostic assay to determine whether they are immune to measles. Because the sensitivity and specificity of commercial measles IgG assays vary, state public health departments can provide information on appropriate second line assays.
NOTE FOR HEALTHCARE PERSONNEL (HCP): CDC has interim guidance that states
Consider vaccinating HCP born before 1957 who do not have other evidence of immunity to measles
During a measles outbreak, 2 doses of measles virus-containing vaccine are recommended for all HCP, regardless of year of birth
See more HCP interim guidance using the CDC link below in ‘Learn More – Primary Sources’
Cannot Readily Show Evidence of Immunity Following Exposure to Measles
MMR vaccine for PEP
Immunoglobulin (IG) for PEP
Prior to Pregnancy
During Pregnancy
Postpartum
Contraindication – Greatly Increases Chance of Serious Adverse Reaction
The following individuals should not receive MMR vaccine
Precaution – Might increase the chance or severity of a serious adverse reaction or might compromise the ability of the vaccine to produce immunity
Precautions for MMR vaccine include the following
CDC: Measles Cases and Outbreaks
CDC: Measles (Rubeola) for Healthcare Professionals
Vaccine Preventable Diseases (aphl.org)
CDC: Routine Measles, Mumps, and Rubella Vaccination
CDC: Interim Infection Prevention and Control Recommendations for Measles in Healthcare Settings
Management of Obstetric–Gynecologic Patients During a Measles Outbreak
Are you an
ObG Insider?
Get specially curated clinical summaries delivered to your inbox every week for free
Please log in to ObGFirst to access this page
OBG Project CME requires a modern web browser (Internet Explorer 10+, Mozilla Firefox, Apple Safari, Google Chrome, Microsoft Edge). Certain educational activities may require additional software to view multimedia, presentation, or printable versions of their content. These activities will be marked as such and will provide links to the required software. That software may be: Adobe Flash, Apple QuickTime, Adobe Acrobat, Microsoft PowerPoint, Windows Media Player, or Real Networks Real One Player.
This educational activity may contain discussion of published and/or investigational uses of agents that are not indicated by the FDA. The planners of this activity do not recommend the use of any agent outside of the labeled indications.
The opinions expressed in the educational activity are those of the faculty and do not necessarily represent the views of the planners. Please refer to the official prescribing information for each product for discussion of approved indications, contraindications, and warnings.
Participants have an implied responsibility to use the newly acquired information to enhance patient outcomes and their own professional development. The information
presented in this activity is not meant to serve as a guideline for patient management. Any procedures, medications, or other courses of diagnosis or treatment discussed or suggested in this activity should not be used by clinicians without evaluation of their patient’s conditions and possible contraindications and/or dangers in use, review of any applicable manufacturer’s product information, and comparison with recommendations of other authorities.
It appears you don't have enough CME Hours to take this Post-Test. We no longer offer Hours.
You are now leaving the ObG website and on your way to PRIORITY at UCSF, an independent website. Therefore, we are not responsible for the content or availability of this site
