Twin pregnancies are followed more closely than singleton pregnancies due to higher risk for complications such as twin-twin transfusion syndrome, selective fetal growth restriction, and preterm labor. Ultrasound is a non-invasive and highly useful tool for screening, diagnosis, and guiding management of these potential complications. Ultrasound monitoring protocols vary between different types of twin pregnancies.
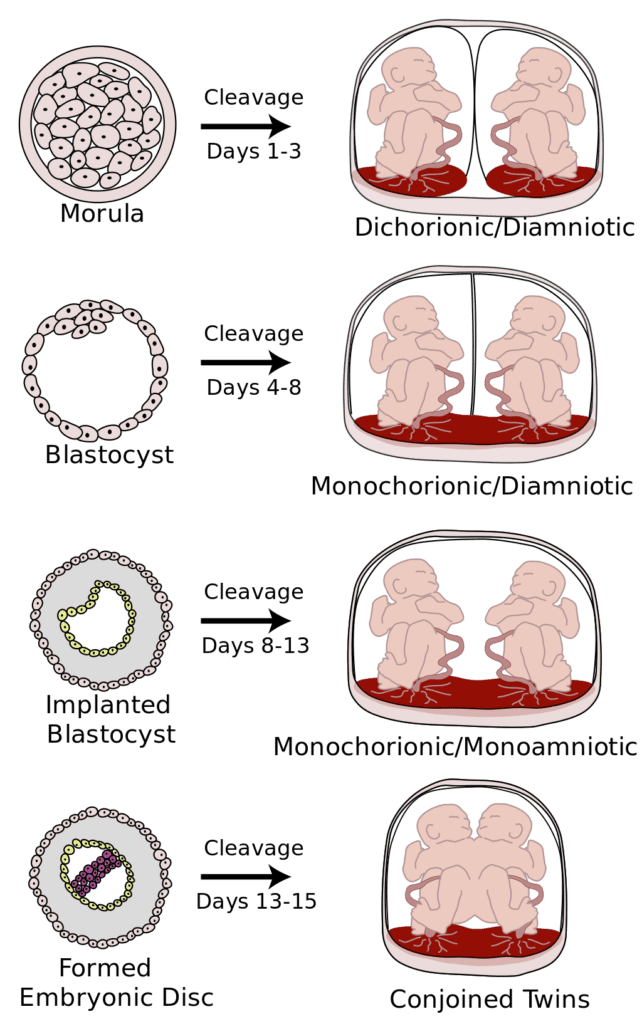
Image by Kevin Dufendach, MD (2008). Used by permission. CC BY 3.0
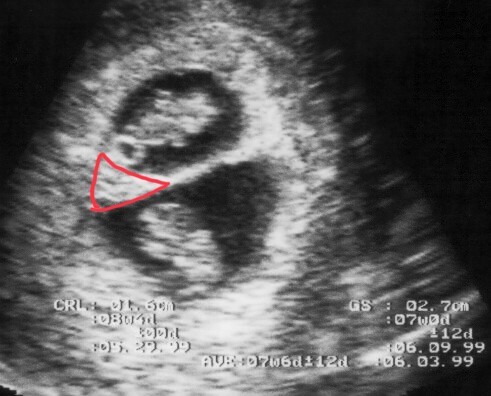
Lambda or Delta Sign Indicating Dichorionic Twins
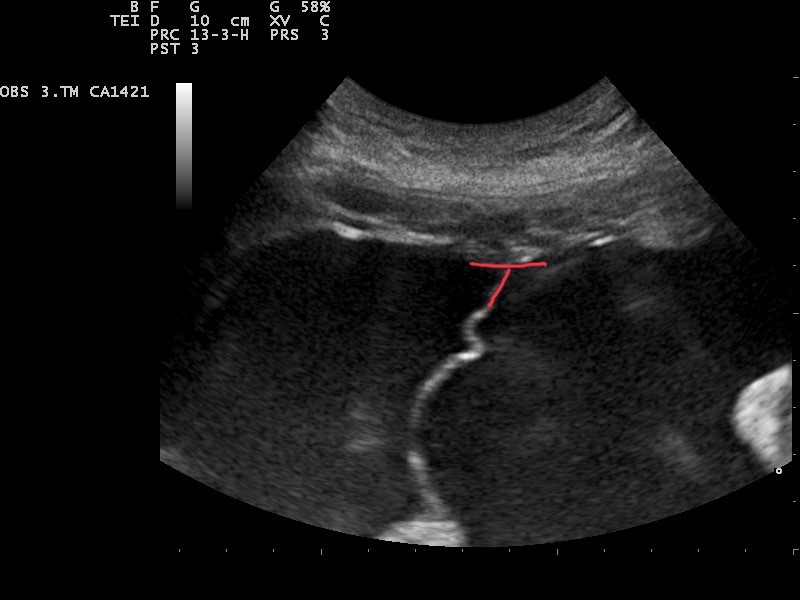
T Sign Indicating Monochorionic Twins
Uncomplicated monochorionic twin pregnancy (see SMFM checklist in ‘Learn More – Primary Sources’ below)
Note: ISUOG guidelines do include umbilical artery Doppler monitoring as part of routine surveillance | ACOG/ SMFM considers evidence to be unclear for uncomplicate monochorionic twins
Uncomplicated dichorionic twin pregnancy
Most twin pregnancies will have good outcomes. However, diligence is required, especially in the case of monochorionic twins due to risk for twin-twin transfusion syndrome (TTTS) and twin anemia polycythemia sequence (TAPS). Monochorionic twins may have potentially significant vascular anastomoses such that the twins share a common vasculature. Significant risks for dichorionic twins include preterm labor, medical complications due to increased placental mass (e.g., preeclampsia and GDM) and selective growth restriction. Different centers will have different protocols for labeling twin A vs twin B. The important point is to be consistent with labeling.
SMFM Special Statement: Updated checklists for management of monochorionic twin pregnancy
ISUOG Practice Guidelines: role of ultrasound in twin pregnancy
ACOG SMFM Committee Opinion 831: Medically Indicated Late-Preterm and Early-Term Deliveries
SMFM Consult Series #72: Twin-twin transfusion syndrome and twin anemia-polycythemia sequence
Maternal Fetal Medicine Specialist Locator-SMFM
Are you an
ObG Insider?
Get specially curated clinical summaries delivered to your inbox every week for free
Please log in to ObGFirst to access this page
OBG Project CME requires a modern web browser (Internet Explorer 10+, Mozilla Firefox, Apple Safari, Google Chrome, Microsoft Edge). Certain educational activities may require additional software to view multimedia, presentation, or printable versions of their content. These activities will be marked as such and will provide links to the required software. That software may be: Adobe Flash, Apple QuickTime, Adobe Acrobat, Microsoft PowerPoint, Windows Media Player, or Real Networks Real One Player.
This educational activity may contain discussion of published and/or investigational uses of agents that are not indicated by the FDA. The planners of this activity do not recommend the use of any agent outside of the labeled indications.
The opinions expressed in the educational activity are those of the faculty and do not necessarily represent the views of the planners. Please refer to the official prescribing information for each product for discussion of approved indications, contraindications, and warnings.
Participants have an implied responsibility to use the newly acquired information to enhance patient outcomes and their own professional development. The information
presented in this activity is not meant to serve as a guideline for patient management. Any procedures, medications, or other courses of diagnosis or treatment discussed or suggested in this activity should not be used by clinicians without evaluation of their patient’s conditions and possible contraindications and/or dangers in use, review of any applicable manufacturer’s product information, and comparison with recommendations of other authorities.
It appears you don't have enough CME Hours to take this Post-Test. We no longer offer Hours.
You are now leaving the ObG website and on your way to PRIORITY at UCSF, an independent website. Therefore, we are not responsible for the content or availability of this site
