Gestational trophoblast disease (GTD) describes a set of diseases originating in placental tissue, specifically the chorionic villi and extravillous trophoblast. GTD includes both benign and malignant conditions. Disorders within GTD are considered distinct entities and are classified as follows
Partial: Karyotype 69XXX or 69XXY or 69XYY
Complete: Karyotype 46 XX (90%) or 46 XY
Serum HCG Plateaus or Rises Following Evacuation of the Uterus
NOTE: Medical complications include the following
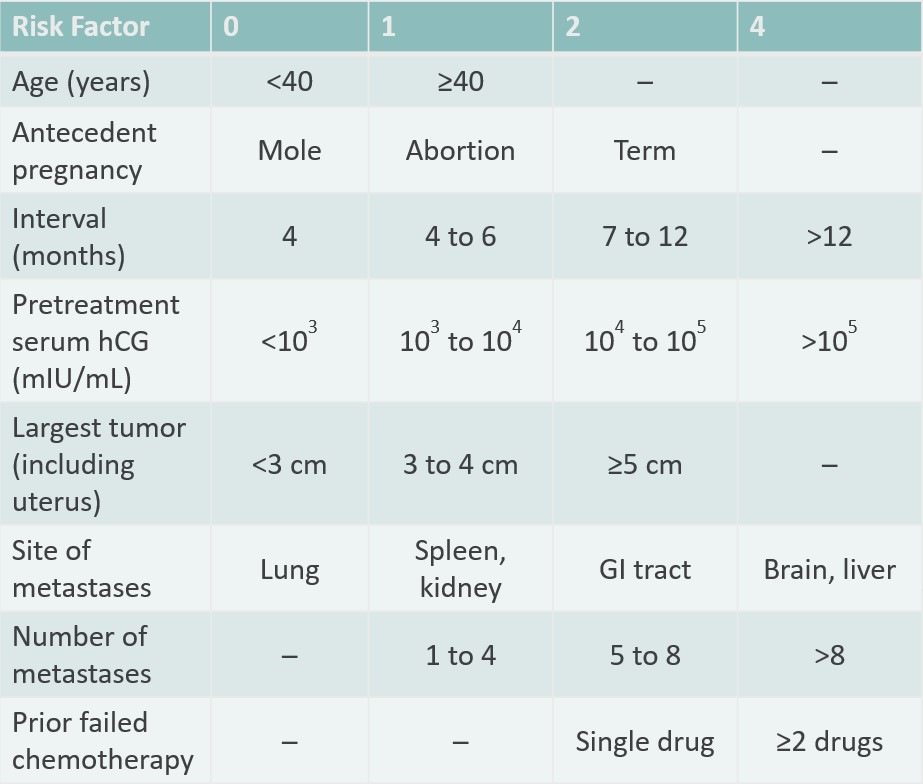
Gestational Trophoblastic Disease Treatment (PDQ®): Health Professional Version
FIGO: Diagnosis and management of gestational trophoblastic disease: 2021 update
Are you an
ObG Insider?
Get specially curated clinical summaries delivered to your inbox every week for free
Please log in to ObGFirst to access this page
OBG Project CME requires a modern web browser (Internet Explorer 10+, Mozilla Firefox, Apple Safari, Google Chrome, Microsoft Edge). Certain educational activities may require additional software to view multimedia, presentation, or printable versions of their content. These activities will be marked as such and will provide links to the required software. That software may be: Adobe Flash, Apple QuickTime, Adobe Acrobat, Microsoft PowerPoint, Windows Media Player, or Real Networks Real One Player.
This educational activity may contain discussion of published and/or investigational uses of agents that are not indicated by the FDA. The planners of this activity do not recommend the use of any agent outside of the labeled indications.
The opinions expressed in the educational activity are those of the faculty and do not necessarily represent the views of the planners. Please refer to the official prescribing information for each product for discussion of approved indications, contraindications, and warnings.
Participants have an implied responsibility to use the newly acquired information to enhance patient outcomes and their own professional development. The information
presented in this activity is not meant to serve as a guideline for patient management. Any procedures, medications, or other courses of diagnosis or treatment discussed or suggested in this activity should not be used by clinicians without evaluation of their patient’s conditions and possible contraindications and/or dangers in use, review of any applicable manufacturer’s product information, and comparison with recommendations of other authorities.
It appears you don't have enough CME Hours to take this Post-Test. We no longer offer Hours.
You are now leaving the ObG website and on your way to PRIORITY at UCSF, an independent website. Therefore, we are not responsible for the content or availability of this site
