
Pregnancy Outcomes after ZIKV Infection in French Territories in the Americas

Healthcare providers should report suspected Zika virus disease cases to their state, local, or territorial health department, who will report to the CDC.
FDA Drug Safety Communication: FDA has reviewed possible risks of pain medicine use during pregnancy

Microcephaly has an incidence of 2 to 12 in 10,000 births in the USA and can be diagnosed prenatally via ultrasound (in second or early third trimester) or postnatally via measurement of head circumference (HC). Microcephaly has been linked to developmental delay, seizures, as well as feeding, vision and hearing problems. Prognosis depends on the severity of the microcephaly and whether it is associated with other anomalies.
SMFM – Fetal Microcephaly
CDC – Postnatal Congenital Microcephaly
American Academy of Neurology and Child Neurology Society
Once fetal microcephaly is identified by ultrasound, fetal magnetic resonance imaging, when available and if potential findings are likely to alter pregnancy management, may be considered.
Fetal magnetic resonance imaging images should be reviewed by a radiologist with expertise in fetal magnetic resonance imaging.
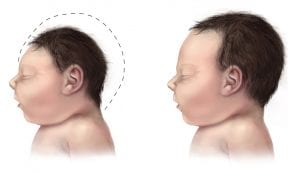
Image credit: Centers for Disease Control and Prevention, National Center on Birth Defects and Developmental Disabilities
Microcephaly: An Epidemiologic Analysis
CDC: Congenital Anomalies of the Nervous System: Microcephaly
SMFM: Ultrasound screening for fetal microcephaly following Zika virus exposure
Practice Parameter: Evaluation of the child with microcephaly (an evidence-based review)
SOGC Guideline 380: Investigation and Management of Prenatally Identified Microcephaly
CDC: Zika Virus For Healthcare Providers
Please log in to ObGFirst to access this page