
Bacterial Vaginosis – CDC Diagnosis and Treatment Recommendations
CLINICAL ACTIONS:
Bacterial Vaginosis (BV) occurs when normal hydrogen peroxide producing Lactobacillus sp. is replaced by an overgrowth of facultative anaerobic bacteria. If a woman presents with symptoms, including vaginal discharge, irritation and malodor
- Diagnose BV if 3 of the following (Amsel) clinical criteria are present
- Homogeneous, thin, white discharge that smoothly coats the vaginal walls
- More than 20% clue cells (e.g., vaginal epithelial cells studded with adherent coccobacilli) on saline microscopic examination
- pH of vaginal fluid >4.5
- Fishy odor of vaginal discharge before or after addition of 10% KOH (i.e., the whiff test), OR
- Gram stain with Nugent scoring is considered the gold standard to diagnose
-
- Used in research settings; impractical for clinicians so Amsel criteria preferred clinically
- Assigns a score to various bacterial concentrations seen on gram stain: 0-3 Normal | 4-6 Intermediate | 7-10 Bacterial vaginosis
- Affirm VP III (Becton Dickinson, Sparks, MD), a DNA hybridization probe test for high concentrations of G. vaginalis
- OSOM BV Blue test (Sekisui Diagnostics, Framingham, MA), which detects vaginal fluid sialidase activity, have acceptable performance characteristics compared with Gram stain
- Nucleic Acid Amplification Tests (NAAT) are also available and “can be used as an alternative to clinical testing in settings where pH paper, KOH, and microscopy are not available”
- Do NOT use Pap tests
- Do NOT culture for G. vaginalis given normal vaginal flora is heterogenous
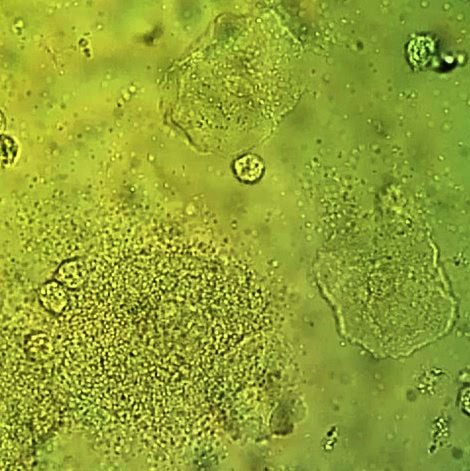
Vaginal wet mount with a NaCl preparation, showing a clue cell at bottom left, and two normal epithelial cells.
SYNOPSIS:
Bacterial Vaginosis (BV) is not caused by a single bacterium, but by high concentrations of facultative anaerobic bacteria (e.g., Prevotella sp. and Mobiluncus sp.), G. vaginalis, Ureaplasma, Mycoplasma, and numerous other anaerobes. BV is associated with multiple or new sex partners, lack of condom use and in particular lack of vaginal lactobacillus. Because BV is not an inflammatory condition, vulvar erythema and edema are not commonly seen, unlike candidiasis and trichomoniasis.
KEY POINTS:
Treatment is recommended for women with symptoms, including discharge, irritation and malodor and may reduce the risk for C. trachomatis, N. gonorrhoeae, T. vaginalis, HIV, and herpes simplex type 2.
Recommended CDC regimens include the following:
- Metronidazole* 500 mg orally twice a day for 7 days OR
- Metronidazole* gel 0.75%, one full applicator (5 g) intravaginally, once a day for 5 days OR
- Clindamycin** cream 2%, one full applicator (5 g) intravaginally at bedtime for 7 days
Alternative Regimens
- Tinidazole* 2 g orally once daily for 2 days OR
- Tinidazole* 1 g orally once daily for 5 days OR
- Clindamycin** 300 mg orally twice daily for 7 days OR
- Clindamycin** ovules 100 mg intravaginally once at bedtime for 3 days
- Secnidazole 2 g orally in a single dose
*Alcohol consumption should be avoided during treatment with oral nitroimidazoles. To reduce the possibility of a disulfiram-like reaction, abstinence from alcohol use should continue for 24 hours after completion of metronidazole and 72 hours after completion of tinidazole.
**Clindamycin ovules use an oleaginous base that might weaken latex or rubber products (e.g., condoms and vaginal contraceptive diaphragms). Use of such products within 72 hours following treatment with clindamycin ovules is not recommended.
- Test all women with BV for HIV and other sexually transmitted diseases (STDs)
- Follow-up visits are unnecessary if symptoms resolve
- Routine treatment of sex partners in not recommended
- Using a different recommended treatment regimen can be considered in women who have a recurrence
- Retreatment with the same recommended regimen is an acceptable approach for treating persistent or recurrent BV after the first occurrence
- Recurrent BV: At least 3 documented, separate episodes of BV in one year
- 0.75% metronidazole gel twice weekly for 4–6 months has been shown to reduce recurrences, although this benefit might not persist when suppressive therapy is discontinued
BV and Preterm Birth
- The USPSTF addresses BV screening during pregnancy and states the following
The USPSTF recommends against screening for bacterial vaginosis in pregnant persons not at increased risk for preterm delivery. (D recommendation)
The USPSTF concludes that the current evidence is insufficient to assess the balance of benefits and harms of screening for bacterial vaginosis in pregnant persons at increased risk for preterm delivery. (I statement)
Learn More – Primary Sources:
Sexually Transmitted Infections Treatment Guidelines 2021
ACOG Practice Bulletin 215: Vaginitis in Nonpregnant Patients
USPSTF: Screening for Bacterial Vaginosis in Pregnant Persons to Prevent Preterm Delivery

Diagnosing Vaginitis – Why the Office Visit Still Matters
CLINICAL ACTIONS:
A patient presents with vaginal inflammation with discharge, pain and/or itching. Next steps should include
Problem-focused history
- BV
- Fishy odor | Thin homogeneous discharge (possibly worse after intercourse)
- Candidiasis
- No odor | White, thick, ‘curdlike’ or ‘cheesy’ discharge | Itching and/or burning
- Trichomoniasis
- Foul odor | Green or yellow, frothy discharge | Vaginal pain or soreness
- Atrophic vaginitis
- Thin, clear discharge | Dryness | Dyspareunia | Itching
- Irritant/allergic vaginitis
- Desquamative Inflammatory vaginitis
- Green or yellow (purulent) discharge | Burning | Dyspareunia
Exam including inspection of the vulva, vagina and cervix
- BV
- Inflammation not usually present
- Candidiasis
- Trichomoniasis
- Inflammation | Strawberry Cervix
- Atrophic vaginitis
- Inflammation | Thin/friable mucosa
- Irritant/allergic vaginitis
- Desquamative Inflammatory vaginitis (DIV)
- Varying vestibular and vaginal erythema
Appropriate laboratory testing
- Collection of and microscopic examination of a 10% KOH and saline prep (wet mount), pH testing and ‘whiff test’ constitute the office-based clinical testing of samples
- Culture (if necessary)
- Yeast: Obtain if recurrent candidiasis or possible non-albicans Candida (suspect if blastospores ‘only’ or persistent treatment after treatment) | Negative microscopy with signs and/or symptoms of candidiasis
- Trichomoniasis: ACOG recommends culture with a negative wet mount in the following circumstances
- Persistent symptoms following treatment | high vaginal pH and WBCs on microscopy | Pap suspicious for T. vaginalis | patient desire for screening
- Note: CDC considers NAAT screening more sensitive for T. vaginalis then culture (previous gold standard) or wet mount
- Mucopurulent cervicitis: Test (DNA or cultures) for gonorrhea or chlamydia
- HSV: If any vulvar fissure/lesion suggestive of herpes simplex virus, perform viral culture or PCR assay for HSV DNA by swabbing the lesion
- Type-specific HSV serologic assays might be useful in the following scenarios: 1) recurrent genital symptoms or atypical symptoms with negative HSV PCR or culture; 2) clinical diagnosis of genital herpes without laboratory confirmation (CDC STD Guidelines)
Perform “Whiff Test” with 10% KOH and Microscopy with Saline
- Positive whiff test
- Negative (-) for WBC: Treat for bacterial vaginosis (BV)
- Positive (+) for WBC: Review signs/symptoms for trichomoniasis or mixed bacterial vaginosis or cervicitis
- Negative whiff test
- negative (-) for WBC: Noninfectious
Determine Vaginal pH
- If pH is normal (<4.7) consider the following
- Infectious: Vulvovaginal candidiasis | Genital herpes
- Noninfectious: Physiologic leukorrhea | Vulvodynia | Dermatitis/dermatoses
If pH is Elevated (>4.7) Consider the Following
- Infectious
- Bacterial vaginosis | Trichomoniasis | Cervicitis
- Noninfectious
- Blood | Semen | Atrophic vaginitis | Lichen planus | Desquamative inflammatory vaginitis (DIV)
SYNOPSIS:
Vaginitis is a general term for disorders of the vagina, but does not indicate the underlying cause. Vaginitis may result from infection, inflammation, or may reflect changes in the normal vaginal microbiome. The disorder is termed vulvovaginosis when the vulva is involved. When patients present with symptoms of itching/burning/irritation/dyspareunia/discharge consider a broad range of possibilities including but not limited to the triad of bacterial vaginosis (BV), trichomoniasis and vulvovaginal candidiasis. Office based tests such as those above also have a low sensitivity. Accurate diagnosis may require a combination of a careful history, vulvar or vaginal biopsy and appropriate culture.
KEY POINTS:
- Self-diagnosis and treatment, while convenient, may be unreliable and results in frequent misuse of OTC products
- FDA approved commercial tests for BV
- ACOG acknowledges that direct DNA probe assays for G vaginalis or chromogenic point-of-care assays for sialidase activity have acceptable performance vs Amsel criteria and Nugent scoring
- However, because these tests only pick up one organism (i.e., G vaginalis) “the diagnostic utility of a test that identifies only a single organism (eg G vaginalis) is still being investigated and is not currently supported”
- No microscope
- Vaginal pH testing narrows the differential diagnosis of vaginitis for BV and trichomoniasis
- Candidiasis: History | Exam | Culture
- Obtain vaginal secretions slide for future Gram stain if possible
- Incidental findings on Pap test
- Not diagnostic
- BV on Pap
- Symptomatic: pH, amine test, and wet mount
- Asymptomatic: Do not treat
- Trichomoniasis on Pap
- High false-positive rate (8% standard and 4% liquid-based)
- Wet mount for confirmation
- If wet mount negative, NAAT or culture
- If diagnostic tests not available, can consider metronidazole, but high rate of unnecessary treatment
Learn More – Primary Sources:
ACOG Practice Bulletin 215: Vaginitis in Nonpregnant Patients
Vaginitis: Diagnosis and Treatment
Advances in Diagnosing Vaginitis: Development of a New Algorithm


