
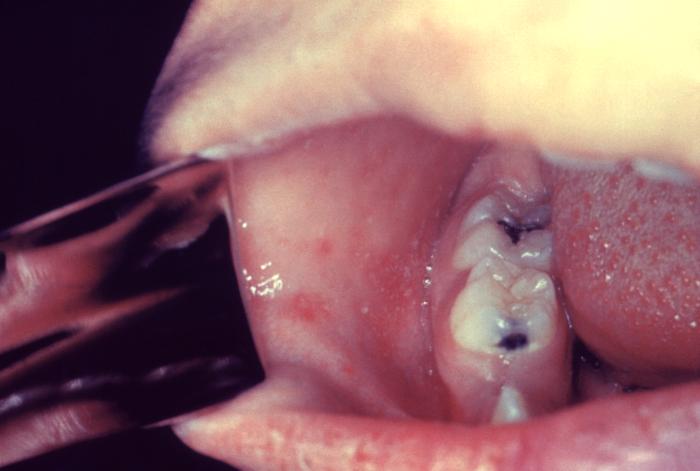
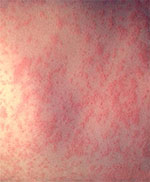
Note: Do not accept verbal reports of immunity
Acceptable presumptive evidence of immunity against measles includes ≥1 of the following
*Note: CDC addresses laboratory evidence of immunity and states the following
*People who have negative or equivocal results for measles IgG should be vaccinated or revaccinated. In some cases it is not possible to vaccinate a patient, and you may need to test them with a second line diagnostic assay to determine whether they are immune to measles. Because the sensitivity and specificity of commercial measles IgG assays vary, state public health departments can provide information on appropriate second line assays.
NOTE FOR HEALTHCARE PERSONNEL (HCP): CDC has interim guidance that states
Consider vaccinating HCP born before 1957 who do not have other evidence of immunity to measles
During a measles outbreak, 2 doses of measles virus-containing vaccine are recommended for all HCP, regardless of year of birth
See more HCP interim guidance using the CDC link below in ‘Learn More – Primary Sources’
Cannot Readily Show Evidence of Immunity Following Exposure to Measles
MMR vaccine for PEP
Immunoglobulin (IG) for PEP
Prior to Pregnancy
During Pregnancy
Postpartum
Contraindication – Greatly Increases Chance of Serious Adverse Reaction
The following individuals should not receive MMR vaccine
Precaution – Might increase the chance or severity of a serious adverse reaction or might compromise the ability of the vaccine to produce immunity
Precautions for MMR vaccine include the following
CDC: Measles Cases and Outbreaks
CDC: Measles (Rubeola) for Healthcare Professionals
Vaccine Preventable Diseases (aphl.org)
CDC: Routine Measles, Mumps, and Rubella Vaccination
CDC: Interim Infection Prevention and Control Recommendations for Measles in Healthcare Settings
Management of Obstetric–Gynecologic Patients During a Measles Outbreak
Please log in to ObGFirst to access this page